If you are reading this article you have probably heard about CBD, and you also maybe know who Scott Gottlieb is and how he feels about CBD.
The Former Food and Drug Administration Commissioner Scott Gottlieb is a man of many titles, he’s a resident fellow at the conservative think tank as well as the American Enterprise Institute, a partner at the venture capital firm New Enterprise Associates, a member of the board of directors of drug maker Pfizer, Inc, and a contributor to the cable financial news network CNBC. So with all that going on, why is he still tweeting negatively about CBD? And should we take what he is saying to heart or with a grain of salt?
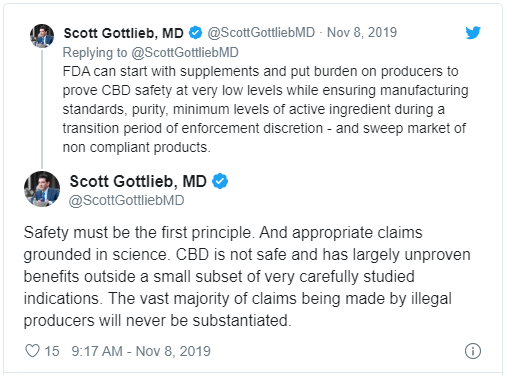
Scott Tweeted out some pretty negative things about CBD:
Scott also stated that “the CBD hype and popularity has outpaced the science” and that “CBD is dangerous, and the benefits that are being claimed are not substantiated”.
What’s more, CBD sales were given a green light in December 2018, when President Trump signed the Farm Bill into law. This legislation legalized the industrial production of hemp, as well as CBD derived from hemp, as long as it doesn’t contain any THC. This gives general retail stores the right to sell CBD products, further broadening their appeal.
However, the pathway to big-time growth for CBD isn’t without its hurdles, and none looms larger than the U.S. Food and Drug Administration (FDA).
Back in march while still head of the fda gottlieb explained that it can typically take 2 – 3 years to develop a regulatory framework for adding a new substance into the food supply. But because cannabis is illicit, and CBD far more complex than conventional additives, it could take the agency even longer to develop regulations and a safety profile for CBD.
Although Gottlieb doesn’t head the FDA anymore, it’s his proactive policies that have shaped the agency into what it is today. When he suggests that it could be a long time before a framework is developed for CBD as an additive, I believe it’s best to take him at his word.
On one hand, established hemp-based CBD product manufacturers with a focus on oils and topicals should be in prime position to deliver sustainable sales growth. On the other side of the coin, it means that CBD stocks are going to have to walk on eggshells to avoid crossing the FDA’s radar.
You see, while hemp-based CBD may have been given a pretty clear sales pathway across the country with the Farm Bill becoming law, adding CBD, a cannabinoid that can be found in marijuana, a still federally illicit substance, to food, beverages, or dietary supplements is still a big no-no. That puts CBD regulation as an additive squarely on the plate of the FDA.
For its part, the FDA was expected to report on the progress of its discussions by the late summer or early fall, according to September tweets from acting Chief Information Officer Amy Abernethy. It’s now November, and the FDA hasn’t provided any updates on where its thinking is headed with regard to CBD being used as an additive.
Nevertheless, this hasn’t stopped former FDA commissioner, Scott Gottlieb, who stepped down from his post earlier this year, from weighing in on CBD.
“Safety must be the first principle. And appropriate claims grounded in science. CBD is not safe and has largely unproven benefits outside a small subset of very carefully studied indications. The vast majority of claims that are being made by illegal producers will never be substantiated.” Scott Said.
We make sure that all of the products that we place on 10bestcbdproducts are verified and safe, the only reason any of these so-called CBD products that Scott is talking about are unsafe is due to their illegitimacy. They’re mainly fake products, and have little to no CBD in them.
